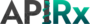Operational Process Summary Diagram
APIRx’s operational process begins with the procurement of cGMP-produced cannabis flower. The next step is to properly clean and extract the specific cannabinoids (THC, CBD, CBG, etc.) according to proprietary extraction methods producing the highest yield and highest purity of individual cannabinoids. These will then be processed into stable APIs utilizing APIRx proprietary methodology to provide the world’s first end-to-end cGMP cannabis APIs and products.
Precise Quality Control Standards
The key objective of APIRx is the production of cGMP natural-cannabinoid-extracted API’s as well as pharmaceutical, nutraceutical and cosmetic products. APIRx possesses the infrastructure, logistics,legal framework,supply of cGMP plant material to produce API’ based on naturally extracted cannabinoids as new API with full DMF and IMPD.
The production of chemical active pharmaceutical ingredients (chem APIs) involves a variety of process steps that must be optimally coordinated. The systems must be implemented flexibly, but cross-contamination of the different APIs must be prevented at the same time. In addition, the effective utilization of production systems that are distributed around the world is becoming increasingly important.
Process optimization is the key to meeting these challenges effectively. The integration of the entire process, from the raw materials to the respective production steps, to the end product, makes it possible to reduce costs and processing times; improve employee safety, including in explosion areas; and prevent contamination of the active ingredients produced. APIRx’s API manufacturing process involves extraction,purification and microencapsulation and adheres to cGMP manufacturing standards.
APIRx is developing products in the fields of wound healing, dermatological diseases, medication related osteonecrosis of the jaws (MRONJ), appetite control and others. Relevant IP applications have been filed and others are being actively developed.
Good Manufacturing Practice (GMP) Certificate
- Defined and controlled manufacturing process
- Clear and non-ambiguous instructions
- Strict monitoring of the production processes
- Rigorous recording of manufacture and distributing
- Writing detailed step-by-step procedures that provide a roadmap for controlled and consistent performance
Food and Drug Administration (FDA) Certificate
The Food and Drug Administration (FDA) is an agency within the U.S. Department of Health and Human Services. The FDA is responsible for protecting the public health by assuring, that medical products, amongst others, are sanitary and properly labeled. APIRx Pharmaceuticals has to pass through several steps in order to get an FDA certificate:
- Toxicity test
- Four testing phases gathering information about APIRx Pharmaceuticals effectiveness and side effects on humans
- FDA review meeting with APIRx Pharmaceuticals
- Review of drug labeling and the facilities where the products will be manufactured
- Approval of the FDA application
European Medicines Agency (EMA) Certificate
The EMA is an agency of the European Union responsible for the evaluation of medicinal products. An EMA certificate of Medicinal Products is issued by the agency on behalf of the European Commission within 10 working days (standard procedure) after receipt of a valid application form.
The aim of the certificate is to support the work of Health Authorities outside the European Union. The certificate confirms the Marketing Authorization status of the medicinal products.The certificates also confirm the Good Manufacturing Practice (GMP) compliance status of the manufacturing sites producing the medicinal product in bulk pharmaceutical form.
Facility Build-Out
APIRx has plans for build-out of a laboratory, manufacturing equipment / lines and office space, including a clean room of the highest quality. Plans also include marketing our cannabinoid pharmaceuticals and branded end products; as well as our state-of-the art technologies towards 3rd parties who are in need of cannabinoid-based API’s as well as final product manufacturing. APIRx products will be regulated as scheduled substances and will require quota for production. These products will be distributed through pharmaceutical distributors and via pharmacists.